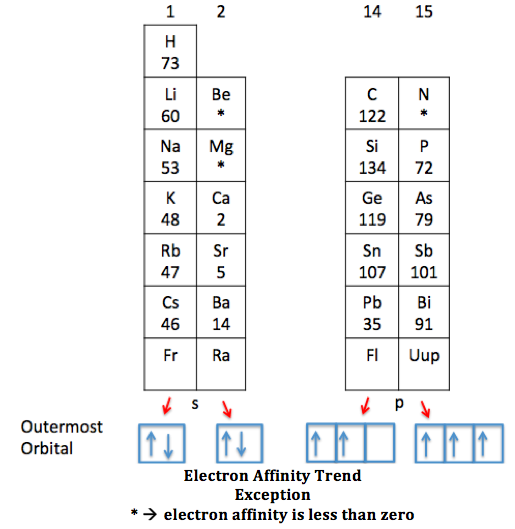
Define Electron Affinity For Chlorine . the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Chlorine gains a stable octet when it captures an. Describe the factors affecting electron affinity and trends within groups. Its high affinity can be attributed to its large atomic radius, or size. use our revision notes to define electron affinity for a level chemistry. chlorine has the highest electron affinity among the elements. electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added.
from study.com
electron affinity of chlorine is 349 kj/mol. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Its high affinity can be attributed to its large atomic radius, or size. Chlorine gains a stable octet when it captures an. Describe the factors affecting electron affinity and trends within groups. use our revision notes to define electron affinity for a level chemistry. chlorine has the highest electron affinity among the elements. In chemistry and atomic physics, the electron affinity of an atom or.
Electron Affinity Definition, Trends & Equation Video & Lesson
Define Electron Affinity For Chlorine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. use our revision notes to define electron affinity for a level chemistry. In chemistry and atomic physics, the electron affinity of an atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Describe the factors affecting electron affinity and trends within groups. chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Chlorine gains a stable octet when it captures an. electron affinity of chlorine is 349 kj/mol.
From brokeasshome.com
Periodic Table Chlorine Electrons Define Electron Affinity For Chlorine chlorine has the highest electron affinity among the elements. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. use our revision notes to define electron affinity for a level chemistry. electron affinity is defined as the change in energy. Define Electron Affinity For Chlorine.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Define Electron Affinity For Chlorine Describe the factors affecting electron affinity and trends within groups. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. In chemistry and atomic physics, the electron affinity of an atom. Define Electron Affinity For Chlorine.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Define Electron Affinity For Chlorine Its high affinity can be attributed to its large atomic radius, or size. use our revision notes to define electron affinity for a level chemistry. electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or. the chlorine atom has the most negative electron affinity of any element, which. Define Electron Affinity For Chlorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Define Electron Affinity For Chlorine the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. use our revision notes to define electron affinity for a level chemistry. electron affinity is. Define Electron Affinity For Chlorine.
From sciencenotes.org
Electron Affinity Trend and Definition Define Electron Affinity For Chlorine electron affinity of chlorine is 349 kj/mol. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Its high affinity can be attributed to its large. Define Electron Affinity For Chlorine.
From brainly.in
Why chlorine has a higher electron affinity than fluorine? Brainly.in Define Electron Affinity For Chlorine Its high affinity can be attributed to its large atomic radius, or size. chlorine has the highest electron affinity among the elements. In chemistry and atomic physics, the electron affinity of an atom or. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. electron affinity of chlorine is 349 kj/mol. Chlorine. Define Electron Affinity For Chlorine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Define Electron Affinity For Chlorine In chemistry and atomic physics, the electron affinity of an atom or. use our revision notes to define electron affinity for a level chemistry. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Its high affinity can be attributed to its. Define Electron Affinity For Chlorine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Define Electron Affinity For Chlorine the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Its high affinity can be attributed to its large atomic radius, or size. electron affinity of chlorine is 349 kj/mol. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron. Define Electron Affinity For Chlorine.
From www.toppr.com
The electron affinity of fluorine is less than that of chlorine because Define Electron Affinity For Chlorine In chemistry and atomic physics, the electron affinity of an atom or. chlorine has the highest electron affinity among the elements. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Chlorine gains a stable octet when it captures an. Its high affinity can be attributed to its large atomic radius, or size.. Define Electron Affinity For Chlorine.
From www.numerade.com
SOLVED What is true of electron affinity? It is equal for sodium and Define Electron Affinity For Chlorine the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. chlorine has the highest electron affinity among the elements. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron. Define Electron Affinity For Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Define Electron Affinity For Chlorine the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. chlorine has the highest electron affinity among the elements. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Describe the factors affecting electron affinity and. Define Electron Affinity For Chlorine.
From www.chegg.com
Solved Electron Affinity Chlorine Flourine Bromine Electron Define Electron Affinity For Chlorine Its high affinity can be attributed to its large atomic radius, or size. electron affinity of chlorine is 349 kj/mol. Describe the factors affecting electron affinity and trends within groups. use our revision notes to define electron affinity for a level chemistry. the chlorine atom has the most negative electron affinity of any element, which means that. Define Electron Affinity For Chlorine.
From askfilo.com
The electron affinity of chlorine is 3.7eV. How much energy in Kcal is re.. Define Electron Affinity For Chlorine Describe the factors affecting electron affinity and trends within groups. electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or. use our revision notes to define electron affinity for a level chemistry. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. . Define Electron Affinity For Chlorine.
From www.numerade.com
SOLVED Write an equation for the second electron affinity of chlorine Define Electron Affinity For Chlorine the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity. Define Electron Affinity For Chlorine.
From ar.inspiredpencil.com
Electron Affinity Define Electron Affinity For Chlorine Its high affinity can be attributed to its large atomic radius, or size. chlorine has the highest electron affinity among the elements. electron affinity of chlorine is 349 kj/mol. the element with the highest electron affinity is chlorine, with a value of 349 kj/mole. electron affinity is defined as the change in energy (in kj/mole) of. Define Electron Affinity For Chlorine.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.1.2 Electron Affinity & Trends of Group 16 Define Electron Affinity For Chlorine the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. chlorine has the highest electron affinity among the elements. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron. Define Electron Affinity For Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Define Electron Affinity For Chlorine In chemistry and atomic physics, the electron affinity of an atom or. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. electron affinity of chlorine is 349 kj/mol. the element with the highest electron affinity is chlorine, with a value. Define Electron Affinity For Chlorine.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Define Electron Affinity For Chlorine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or. Chlorine gains a stable octet when it captures an. chlorine has the highest electron affinity among the elements. use our revision. Define Electron Affinity For Chlorine.